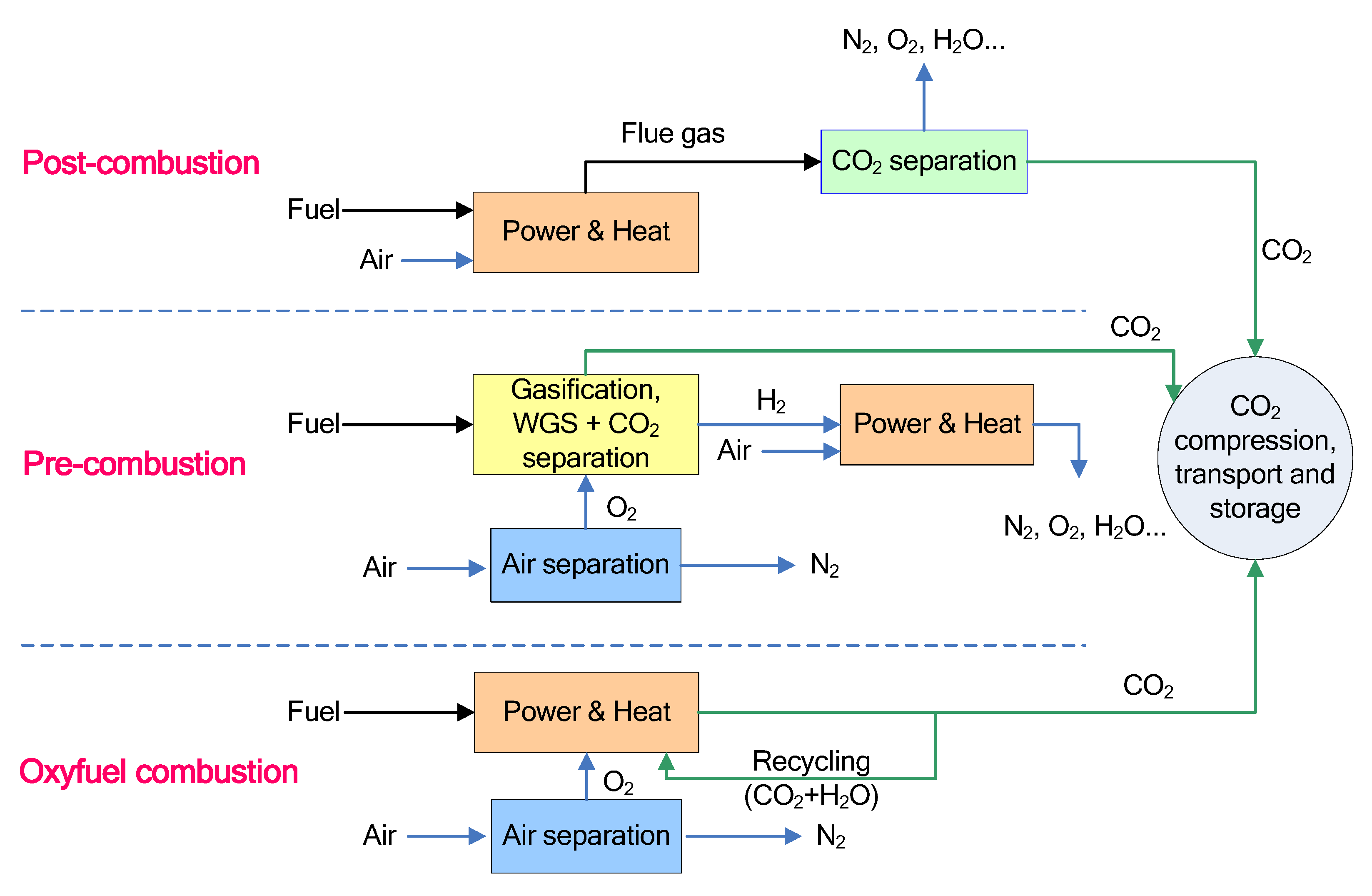
Membrane Gas Separation .pdf
Posted : adminOn 3/12/2018View Notes - Week 6. Membrane gas separation.pdf from CEIC 8341 at University of New South Wales. CEIC8341 Membrane Processes Membrane Gas Separation School of. 30 Years of Membrane Technology for Gas Separation Paola Bernardo. Figure 1: Milestones in the industrial application of membrane gas separation systems. Download full-text PDF. Membrane gas separation technologies for. A discussion on the different materials used to produce membranes for gas separation is. Membrane Gas Separation YURI YAMPOLSKII A.V. Topchiev Institute of Petrochemical Synthesis, Moscow, Russia BENNY FREEMAN University of Texas at Austin, Austin, TX, USA.

Membrane cartridge While polymeric membranes are economical and technologically useful, they are bounded by their performance, known as the Robeson limit (permeability must be sacrificed for selectivity and vice versa). This limit affects polymeric membrane use for CO 2 separation from flue gas streams, since mass transport becomes limiting and CO2 separation becomes very expensive due to low permeabilities. Membrane materials have expanded into the realm of,,, and due to their strong thermal and chemical resistance as well as high tunability (ability to be modified and functionalized), leading to increased permeability and selectivity. Can be used for separating gas mixtures where they act as a permeable barrier through which different compounds move across at different rates or not move at all. The membranes can be nanoporous, polymer, etc. And the gas molecules penetrate according to their size,, or solubility.
Microscopic model of a nanoporous membrane. The white open area represents the area the molecule can pass through and the dark blue areas represent the membrane walls. The membrane channels consists of cavities and windows. The energy of the molecules in the cavity is U c and the energy of a particle in the window is U w. Nanoporous membranes are fundamentally different than polymer-based membranes in that their chemistry is different and that they do not follow the Robeson limit for a variety of reasons. The simplified figure of a nanoporous membrane shows a small portion of an example membrane structure with cavities and windows.
The white portion represents the area where the molecule can move and the blue shaded areas represent the walls of the structure. In the engineering of these membranes, the size of the cavity (L cy x L cz) and window region (L wy x L wz) can be modified so that the desired permeation is achieved. It has been shown that the permeability of a membrane is the production of adsorption and diffusion. In low loading conditions, the adsorption can be computed by the Henry coefficient. Corel Draw X4 Serial Number And Activation Code 2013. If the assumption is made that the energy of a particle does not change when moving through this structure, only the entropy of the molecules changes based on the size of the openings. If we first consider changes the cavity geometry, the larger the cavity, the larger the entropy of the absorbed molecules which thus makes the Henry coefficient larger.
For diffusion, an increase in entropy will lead to a decrease in free energy which in turn leads to a decrease in the diffusion coefficient. Conversely, changing the window geometry will primarily effect the diffusion of the molecules and not the Henry coefficient. In summary, by using the above simplified analysis, it is possible to understand why the upper limit of the Robeson line does not hold for nanostructures. In the analysis, both the diffusion and Henry coefficients can be modified without effecting the permeability of the material which thus can exceed the upper limit for polymer membranes. Silica Membranes [ ] Silica membranes are and can be made with high uniformity (the same structure throughout the membrane). The high porosity of these membranes gives them very high permeabilities.
Synthesized membranes have smooth surfaces and can be modified on the surface to drastically improve selectivity. Matlab 7. Six Organs Of Admittance The Sun Awakens Rar. 5 Free Download Full Version here. Functionalizing silica membrane surfaces with amine containing molecules (on the surface groups) allows the membranes to separate CO 2 from flue gas streams more effectively. Surface functionalization (and thus chemistry) can be tuned to be more efficient for wet flue gas streams as compared to dry flue gas streams.
While previously, silica membranes were impractical due to their technical scalability and cost (they are very difficult to produce in an economical manner on a large scale), there have been demonstrations of a simple method of producing silica membranes on hollow polymeric supports. These demonstrations indicate that economical materials and methods can effectively separate CO 2 and N 2. Ordered mesoporous silica membranes have shown considerable potential for surface modification that allows for ease of CO 2 separation. Surface functionalization with leads to the reversible formation of (during CO 2 flow), increasing CO 2 selectivity significantly. Zeolite Membranes [ ]. A typical zeolite. Thin layers of this crystalline zeolite structure can act as a membrane, since CO 2 can adsorb inside of the pores.